Synthesis of Cu3(MoO4)2(OH)2 nanostructures by simple aqueous precipitation: understanding the fundamental chemistry and growth mechanism
Abstract
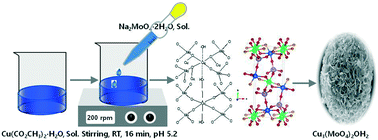
Lindgrenite (Cu3(MoO4)2(OH)2) nanoflowers were synthesized through the simplest possible route by an aqueous chemical precipitation technique at room temperature without using any surfactants, template, expensive chemicals, complex instrumentation or tedious multistage synthesis process. Their morphology, structure, thermal properties, surface area, synthesis chemistry, and structural and growth mechanisms involved in the synthesis process were analyzed. Using XRD, FE-SEM, HR-TEM and FT-IR spectroscopy, their structure and morphology were analyzed. The thermal stability, surface area and porosity of the Cu3(MoO4)2(OH)2 nanoflowers were analyzed by TGA and BET. XRD analysis showed that the Cu3(MoO4)2(OH)2 nanoflowers have a pure monoclinic structure. The morphological analysis showed that the Cu3(MoO4)2(OH)2 nanoflowers are ∼10 μm in size, which are formed from self-assembly of thin nanosheets with a thickness of ∼20 nm. TGA indicated that the Cu3(MoO4)2(OH)2 nanoflowers are stable materials up to 328 °C and the isotherm from BET analysis indicated that the Cu3(MoO4)2(OH)2 nanoflowers are non-porous materials. The BET surface area of the synthesized Cu3(MoO4)2(OH)2 nanoflowers was found to be 21.357 m2 g−1. Moreover, the effects of the pH value and reaction time on the morphology of the Cu3(MoO4)2(OH)2 nanoflowers were studied and their optimization was performed. The results of the optimization study indicated that the reaction time and pH are two important parameters influencing the nucleation, growth, morphology, and synthesis mechanism. These flower-shaped Cu3(MoO4)2(OH)2 nanostructures are promising precursors for preparing molybdenum oxide materials which have various applications and can be synthesized in a very simple one-pot reaction system using commonly available chemicals without using a complex route.


 Please wait while we load your content...
Please wait while we load your content...