Reversible water uptake by a porous molecular crystal from metal complex of gemini surfactant†
Abstract
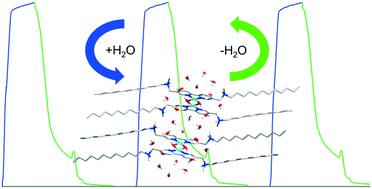
We present a novel porous molecular crystal (PMC) constructed using metal complexes, which shows a striking feature, namely that such a crystal exhibits a reversible transition in the crystal structure when subjected to water removal/uptake cycles. It was found that the dehydrated crystal adsorbed a given quantity of water, that is, each complex assembling block, derived from two gemini surfactant molecules with a bipyridyl spacer and two copper ions (Cu2+) coordinated, stuck to 10 molecules of water. Also, the water included in crystals might generate a unique two-dimensional (2D) hydrogen bond network, including metal–ligand complex units, counter ions and crystalline water. Specially, the crystalline structure was not destroyed upon desolvation, which is ascribed to the metal ion introduced as well as the counter ion (Br−) providing additional electrostatic interactions and hydrogen bonds. Our findings indicate that the introduction of metal ions may provide an alternative to the common weak interactions when designing PMCs. In addition, this crystal material is sensitive to moisture, extending the scope of such a gemini species for potential applications in chemical sensors and in humid environments.


 Please wait while we load your content...
Please wait while we load your content...