Growth of idiomorphic LiMnPO4 crystals in molten NaCl–KCl and LiF–NaCl–KCl fluxes†
Abstract
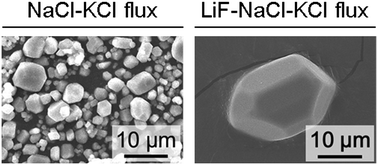
Olivine-type idiomorphic polyhedral LiMnPO4 crystals grown from a molten LiF–NaCl–KCl ternary flux at 700 °C are found to be surrounded with well-defined facets of major {100}, {101}, and {210} faces with minor {011} faces. The average size of these crystals of ∼20.7 μm was around ten times larger than those grown from a molten NaCl–KCl binary flux. In situ XRD and TG-DTA measurements revealed that LiMnPO4 crystals grew from the melt during heating and holding at 700 °C with little assistance from the supersaturation produced by flux evaporation. Time-dependent scanning electron microscopy observation found that Ostwald ripening caused by repeated partial dissolution and reprecipitation of the LiMnPO4 crystals is the dominant factor in both the development of crystal facets and the mean diameter of individual crystals. The addition of LiF is therefore considered to promote the development of well-defined polyhedral crystals by increasing the solubility of LiMnPO4 in the molten flux.

 Please wait while we load your content...
Please wait while we load your content...