Anion hydrogen bonding from a ‘revealed’ urea ligand†
Abstract
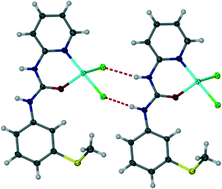
Hydrogen bonding from a urea group to hydrogen bond acceptor anions can adopt either R12(6) or R22(8) motifs depending on the proximity of hydrogen bond acceptor atoms. However, for the sterically bulky and weaker hydrogen bond acceptor triflate anion, hydrogen bond acceptor polymorphism is observed.

 Please wait while we load your content...
Please wait while we load your content...