Solvothermal synthesis of coordination polymers at different temperatures and their luminescence studies†
Abstract
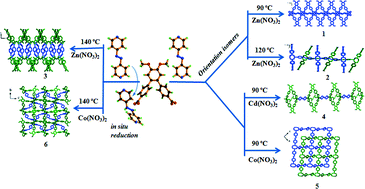
A new linker, [1,1′:2′,1′′-terphenyl]-4′,5′-dimethoxy-4,4′′-dicarboxylic acid (H2L) has been used along with 4,4′-azobispyridine (azpy) as the co-linker to solvothermally synthesize six coordination polymers (CPs). These compounds are formulated as {[Zn(L)(azpy)0.5]·(0.5H2O)}n (1), {[Zn2(L)2(azpy)]·(DMF)·(1.5H2O)}n (2), {[Zn(L)(bphy)]·(DMF)}n (3), [Cd(L)(azpy)0.5(DMF)(H2O)]n (4), {[Co(L)(azpy)](2H2O)}n (5) and {[Co3(L)2(bphy)2(HCOO)2(H2O)2](6DMF)(6H2O)}n (6) (bphy = 1,2-bis(4-pyridyl)hydrazine). Interestingly, 1 and 2 which are synthesized at 90 and 120 °C, respectively, are found to be orientation isomers. When the solvothermal reactions are carried out at 140 °C, the azo-bond in the co-linker azpy is reduced to bphy as found in 3 and 6. All the complexes exhibit an sql topology. They are characterized by X-ray crystallography, elemental analysis, thermogravimetry, powder X-ray diffraction and infrared spectroscopy. Solid state photoluminescence studies show an intra-ligand π–π* transition in each case.

 Please wait while we load your content...
Please wait while we load your content...