A detailed comparative structural study of the hydrogen bonded networks in solids, obtained by the reaction of 4,4′-bipyridine and varied alkane-α,ω-diphosphonic acids†
Abstract
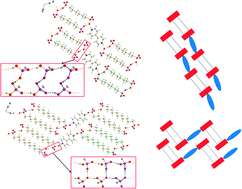
In a comparative structural study, the solid state structures of seven new compounds obtained by the reaction of 4,4′-bipyridine and alkane-α,ω-diphosphonic acids with different chain lengths have been characterised by single crystal X-ray diffraction and vibrational spectroscopy. The compounds are composed of either [4,4′-Hbipy]+ or [4,4′-H2bipy]2+ cations and [H2O3P(CH2)nPO3H]− anions and exhibit layered structures with clearly separated anionic and cationic areas. Within the three [4,4′-Hbipy]+ salts, the 4,4′-bipyridin-1-ium cations are connected to adjacent ones by medium strong, charge supported N+–H⋯N hydrogen bonds to form infinite chains. The anionic substructures of these compounds are quite different. The crystal structures of the five [4,4′-H2bipy]2+ salts consist of anionic, hydrogen bonded strands which are linked to adjacent ones by 4,4′-bipyridine-1,1′-diium cations. All but one can be broken down into two different types of structures in which the cations either lie in line with the anionic strands (type 1) or perpendicular to the aforementioned (type 2). For [4,4′-H2bipy][H2O3P(CH2)6PO3H]2 (2), two concomitant polymorphs have been obtained and characterised by single crystal and powder X-ray diffraction. Both polymorphs were classifiable into type 1 and 2, respectively. Only [4,4′-H2bipy][H2O3P(CH2)9PO3H]2 (4) is a three-dimensional hydrogen bonded framework and therefore not assignable to one of these two types of structures. π-System distances and a remarkable bending of the alkylene chains document the contribution of π–π-stacking and van der Waals interactions to the lattice enthalpy.

 Please wait while we load your content...
Please wait while we load your content...