Polymorphism control in the mechanochemical and solution-based synthesis of a thermochromic Schiff base†
Abstract
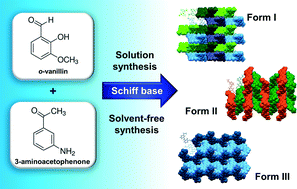
Three crystal forms of a thermochromic Schiff base, namely 1-{3-[(2-hydroxy-3-methoxy-benzylidene)-amino]-phenyl}-ethanone, derived from o-vanillin and 3-aminoacetophenone, were obtained by conventional solution-based methods. Two out of the three polymorphs were synthesized by mechanochemical syntheses, under solvent-free conditions. Herein, we report a study of the dependence of the composition of solvent, the crystallization temperature and impurities on the outcome of the synthesis of Schiff base polymorphs. We report the important role of seed crystals in directing the supramolecular organization of the product of a covalent solvent-free reaction towards the intended polymorphic outcome as well. All obtained polymorphs were investigated by means of thermal analysis, single crystal X-ray diffraction, ex situ and in situ powder X-ray diffraction and IR spectroscopy. The polymorphs display interesting and remarkably different molecular packing arrangements governed by C–H⋯O interactions leading to two-dimensional networks in forms I and III, and a three-dimensional networks in form II.
- This article is part of the themed collection: Editor’s Collection: Mechanochemistry

 Please wait while we load your content...
Please wait while we load your content...