Control over crystallization of CaCO3 micro-particles by a novel CO2SM†
Abstract
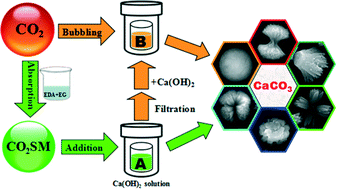
Different morphologies of CaCO3 micro-particles through a hydrothermal reaction between Ca(OH)2 saturated limpid solution and a new CO2-storage material (CO2SM), which was obtained from an equimolar system 1,2-ethylenediamine (EDA) + 1,2-ethylene glycol (EG) uptaking CO2, were presented without any additives. It's worth noting that the morphologies of the CaCO3 precipitates could be controlled as uniform sheaf-like (pure calcite) at the lower CO2SM concentration (0.6 g L−1) and homogeneous spherical-like (pure vaterite) at the higher CO2SM concentration (40 g L−1) for 1 h at 100 °C, in which the released EDA and/or EG from CO2SM had unexpected functions as surfactants. After the precipitation of CaCO3 crystals in 40 g L−1 CO2SM solution, the filtered solution could not only be reused to absorb CO2, but also to prepare the same crystal phase CaCO3 micro-particles repeatedly with the addition of Ca(OH)2. As a result, this novel synthesis process of CaCO3 micro-particles with CO2SM offers an alternative way for the comprehensive utilization of CO2.

 Please wait while we load your content...
Please wait while we load your content...