Fe2O3 icositetrahedra: evolution and their comparative photocatalytic activities†
Abstract
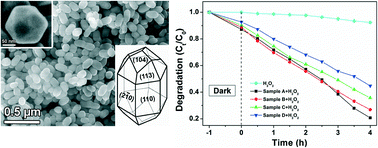
Single crystal Fe2O3 icositetrahedra have been synthesized by a facile hydrothermal synthesis method in the presence of dihydrogen phosphate ions. The icositetrahedron particles are of hexagonal shape and enclosed by 24 quadrilaterals. It could be seen as a particle with six equivalent {110} facets added to the truncated hexagonal bipyramidal structure, that is, it is enclosed by six {110} planes, six {104} planes and twelve {113} planes. Dihydrogen phosphate ions play an important role in the formation of α-Fe2O3 icositetrahedron particles, because high concentration of [H2PO4]− drives the ferric oxide nanocrystals growth along the [006] zone axis. Furthermore, the as-synthesized Fe2O3 icositetrahedron single crystals show comparative photocatalytic activities on the degradation of cationic dye Rhodamine B, which make them promising candidates for catalysts and sensing materials.

 Please wait while we load your content...
Please wait while we load your content...