Hundreds of milligrams of Cr(vi) adsorbed on each gram of Cu2O@Cu fractal structures: kinetics, equilibrium, and thermodynamics†
Abstract
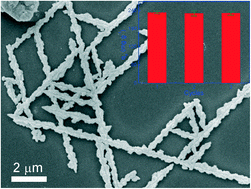
In this study, branch-like Cu2O@Cu fractal structures were prepared by a facile urea-assisted solvothermal procedure. Each branch of the fractal had its own branch and outstretched along a direction of 60°. On the basis of the Langmuir isotherm model, the maximum Cr(VI) adsorptive capacities on these fractal structures were estimated to be 360, 520, 460, and 760 mg g−1 at 293, 298, 303, and 308 K, respectively. These values, to our knowledge, were much larger than those reported for metal oxide adsorbents in previous literature. More strikingly, with the help of a pseudo-second-order model, the initial Cr(VI) adsorption rate was estimated to be 32 mg g−1 min−1, which was significantly higher than those reported for many other adsorbents. Thermodynamic analyses of enthalpy, entropy and Gibbs free energy further demonstrated that these outstanding kinetics and equilibrium results were mainly due to a spontaneous quasi-chemical adsorption process, rather than the impacts of the specific surface area and volume density of our fractal adsorbents.

 Please wait while we load your content...
Please wait while we load your content...