Crystal growth and characterization of solvated organic charge-transfer complexes built on TTF and 9-dicyanomethylenefluorene derivatives†
Abstract
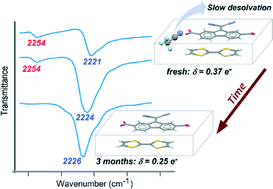
A series of 1 : 1 organic complexes have been synthesized by reaction between TTF (tetrathiafulvalene) and three 9-dicyanomethylenefluorene derivatives: 9-dicyanomethylene-2,7-dinitrofluorene (DDF), 9-dicyanomethylene-2,4,7-trinitrofluorene (DTF) and 9-dicyanomethylene-4,5,7-trinitrofluorene-2-carboxylic acid (DC2TF). The following formulas were determined by X-ray diffraction and elemental analysis for these complexes: (TTF-DDF)·CH3CN (1), (TTF-DDF)·0.5PhCl (2), (TTF-DTF)·CH3CN (3), (TTF-DTF)·0.5Me2CO (4) and (TTF-DC2TF)·H2O (5). A sixth solvated compound was also obtained, with a different stoichiometry, (TTF)3(DC2TF)2·2CH3CN (6). The degree of charge transfer in 1–5 was estimated by IR and Raman spectroscopy. The lattice solvent, acetonitrile, chlorobenzene, or acetone is slowly released from the crystals of complexes 1–4, inducing a significant decrease in charge transfer over time. These crystals converge over months towards materials close to the neutral state. Hydrate 5 is air-stable, and displays a degree of charge transfer, δ = 0.48 e−, close to the range of semiconducting or metal–organic complexes. Finally, compound 6 is an ionic crystal, and is thus expected to be an insulating material.


 Please wait while we load your content...
Please wait while we load your content...