Exploration of CH⋯π interactions involving the π-system of pseudohalide coligands in metal complexes of a Schiff-base ligand†
Abstract
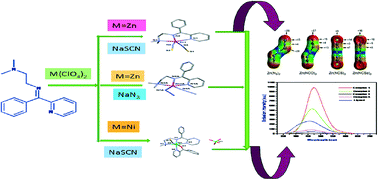
A series of four mononuclear Schiff-base complexes, namely, [Zn(L)(NCS)2] (1), [Zn(L)(N3)2] (2), [Cu(L)(NCS)2] (3) and [Ni (L)(2bpy)(NCS)](ClO4) (4), [where L = N,N-dimethyl-N′-(phenyl-pyridin-2-yl-methylene)-ethane-1,2-diamine and 2bpy = 2-benzoylpyridine] were synthesized with the aim of investigating the role of different non-covalent weak interactions responsible for the crystal packing of the complexes. All of them were structurally characterised by X-ray diffraction analysis. In addition to conventional CH3⋯π and π⋯π interactions, the importance of unconventional C–H⋯π interactions in the crystal packing of compounds 1–4 was investigated by means of Hirshfeld surface analysis and DFT calculations. In these unconventional C–H⋯π interactions, the π-system (electron donor) is provided by the pseudohalide coligands. The interactions formed by the π-system depend on the nature of the pseudohalide (N3, NCO, NCS or NCSe) as demonstrated by molecular electrostatic potential calculations. Additionally, we have explored the photophysical properties of these complexes. Finally, we have combined a search in the Cambridge Structural Database and DFT energy calculations to analyse the rare ambidentate behaviour of SCN within the same complex.


 Please wait while we load your content...
Please wait while we load your content...