A facile one-pot hydrothermal synthesis of branched α-MnO2 nanorods for supercapacitor application
Abstract
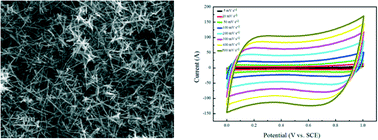
Branched α-MnO2 nanorods are synthesized using a facile hydrothermal method without surfactants or templates. The formation of α-MnO2 with different morphologies, including branched nanorods and nanorods with controllable length, is achieved by controlling the starting concentration of reactants. The morphology and structure of the branched α-MnO2 nanorods are fully characterized and the growth mechanism is proposed based on the experimental observation. The novel structures presented here enrich the nanoscale community of α-MnO2 materials, thus enabling greater potential applications. The electrochemical properties of as-synthesized branched α-MnO2 nanorods are also studied by cyclic voltammetry (CV) and galvanostatic charge/discharge measurement. The branched α-MnO2 nanorod electrode shows a high specific capacitance of 182 F g−1 at a current density of 2 A g−1, with a good rate capability (72.5% at 64 A g−1) and excellent cycling stability.

 Please wait while we load your content...
Please wait while we load your content...