Pristine hollow microspheres of Mn2O3 as a potential anode for lithium-ion batteries†
Abstract
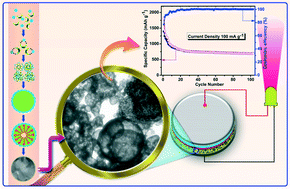
Hollow Mn2O3 microspheres have been synthesized by a simple and easy-to-adopt solvothermal method, facilitated by ethanol to obtain the phase pure product at 600 °C. Herein, the ascorbic acid aided inside-out Ostwald ripening promotes the formation of porous microspheres with a hollow interior surface, which is desirable to allow faster transport of lithium ions and to accommodate the volume changes associated with the Mn2O3 anode, especially upon extended cycling. Further, an appreciable high surface area of 426.6 m2 g−1 has been calculated from BET analysis, which is in favour of imparting excellent electrochemical properties that include high reversible capacity, good cycling stability and acceptable rate capability. Interestingly, Mn2O3 hollow microspheres exhibit 425 mA h g−1 under the influence of 1 A g−1 current density, thus qualifying itself as a high rate anode material, bestowed with the added advantage of appreciable high capacity, extractable under nominal current densities. For instance, without requiring the formation of composite, the as-received Mn2O3 anode delivers a steady-state capacity of 760 mA h g−1 at 50 mA g−1 and 610 mA h g−1 at 100 mA g−1, especially after completing 100 cycles. The synergistic advantage of the microspheres containing hierarchically arranged nanostructures and the hollow interior nature with better uptake of the electrolyte results in obtaining promising electrochemical performance from the currently synthesized Mn2O3 anode.

 Please wait while we load your content...
Please wait while we load your content...