Alloyed CuInS2–ZnS nanorods: synthesis, structure and optical properties†
Abstract
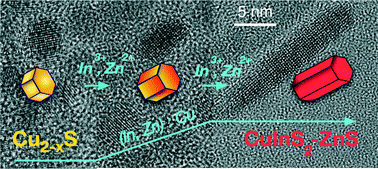
Alloyed CuInS2–ZnS nanocrystals are promising candidates for application in biolabeling, photocatalysis, solar energy conversion, and light emitting diodes. When charge transport is of importance, elongated nanoparticles are advantageous, because of their higher electrical conductivity compared to the quasi-spherical ones. However, still little is known about the growth mechanism of such nanostructures composed of quaternary materials. Here, CuInS2–ZnS nanorods were synthesized by a heating-up method, and their Zn content was controlled by changing the composition of the reaction solution. A mixture of oleylamine and oleic acid is used as solvent. Copper, indium, and zinc acetate are the sources of the cations, while sulfur monomers stem from the thermal decomposition of tert-dodecanethiol. The growth of CuInS2–ZnS nanorods starts with the formation of copper sulfide particles. They are gradually converted to CuInS2–ZnS by incorporation of indium and zinc ions. Alloyed CuInS2–ZnS nanorods are the only product, independent of the amount of zinc applied; Raman spectroscopy measurements show no separate ZnS phase. At longer reaction time, the nanorods aggregate to form dimers. The onset of the absorption and the position of the maximum of the emission as well as the fluorescence lifetime depend on the composition of the nanorods.

 Please wait while we load your content...
Please wait while we load your content...