Effect of the crystal plane figure on the catalytic performance of MnO2 for the total oxidation of propane
Abstract
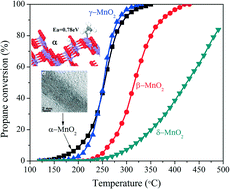
Nanosized MnO2 particles with α-, β-, γ-, and δ-crystal phases were synthesized hydrothermally, and characterized by XRD, SEM, HR-TEM, low temperature N2 adsorption, TPR, TPD, FT-IR and Raman spectroscopy. The density functional theory (DFT) method was used to calculate the adsorption of propane and O2 on MnO2 catalysts. The effect of the crystal phases or crystal plane figure on the catalytic properties of MnO2 for the total oxidation of propane was evaluated. The results showed that α-, β- and γ-MnO2 exhibited a 1D structure, and δ-MnO2 was a 2D layered-structure material. For deep oxidation of propane, the catalytic activities of the MnO2 samples decreased in the order of α- ≈ γ- > β- > δ-MnO2. Compared with the other three MnO2 samples, α-MnO2 exhibited the highest catalytic activity and stability for propane oxidation, its T10 and T90 were 204 °C and 290 °C, respectively. For the different crystal phases of MnO2, there are distinct differences in the chemical bonds (Mn–O–Mn and Mn–O) and linking modes of [MnO6] octahedra, the adsorption energies of propane on the surface of MnO2 are varied in the order of (310) facet of α- > (120) of γ- > (110) of β- > (001) of δ-MnO2, and the presence of translational motion in α-MnO2 along with its stronger deformation and stretching modes may lead to its better catalytic activity for propane oxidation.

 Please wait while we load your content...
Please wait while we load your content...