Crystallographic and computational investigation of intermolecular interactions involving organic fluorine with relevance to the hybridization of the carbon atom†‡
Abstract
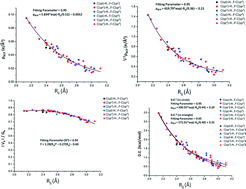
The characteristics of the C(sp,sp2,sp3)–H⋯F–C(sp,sp2,sp3) intermolecular interactions present in molecular crystals on the basis of the hybridization of the carbon atom in the interaction have been analyzed. The Cambridge Structure Database has been extensively searched for the existence of such interactions as a function of the different combinations of hybridization possible for C–H⋯F–C interactions. The parameters in the search involve restriction with the following limits: 2.1 Å < H⋯F distance < 3.0 Å and 110° < C–H⋯F angle < 180°. PIXEL calculations performed on selected molecular pairs showed that C–H⋯F interactions are mainly of a dispersive nature. In molecules involving the presence of a C–H donor atom wherein the carbon exists in sp hybridization, preferential electrostatic contribution was observed. A full topological analysis using the QTAIM approach confirms the presence of a BCP in all the extracted molecular pairs in the crystal geometry, thereby confirming the presence of the C–H⋯F interaction regardless of the hybridization of the participating atoms. Both the electron density (ρ) and the Laplacian (∇2ρ) evaluated at the BCP showed exponential dependence on the bond path length for all the existing interactions.

 Please wait while we load your content...
Please wait while we load your content...