Effect of anions on the self-assembly of two Cd–organic frameworks: syntheses, structural diversity and photoluminescence properties†
Abstract
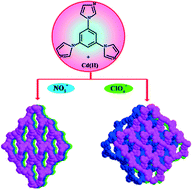
By employing a rigid tripodal ligand 1,3,5-tris(1-imidazolyl)benzene (tib) to assemble with two different Cd(II) salts, we have obtained two distinct cationic metal–organic frameworks, [Cd(tib)2·2NO3·4DMF]n (1) and [Cd(tib)2·2ClO4·4DMF]n (2) (DMF = N,N-dimethylformamide). The compounds were structurally characterized by single-crystal X-ray diffraction analyses and further characterized by infrared spectra (IR), elemental analyses, powder X-ray diffraction (PXRD), and thermogravimetric analyses (TGA). Both compounds 1 and 2 are constructed based on 2D square lattice (sql) nets, through ligand-to-axial (L–A), pillared by triangular tib ligands, resulting in two diverse 3D pillar-layered architectures. Compound 1 features a cationic framework with a (3,6)-connected rtl topology and has rhomboid channels; while for 2, the 2D layers repeat in an ⋯ABAB⋯ staggered stacking mode pillared by tib ligands, resulting in a different cationic framework from 1 with a pyr net. The results demonstrate that counterions NO3− and ClO4− play an important role in the fabrication of structures of 1 and 2. Furthermore, the photoluminescence of compounds 1 and 2 have been investigated in the solid state at ambient temperature.

 Please wait while we load your content...
Please wait while we load your content...