Manipulation on ZnO heterostructures: from binary ZnO–Ag to ternary ZnO–Ag–polypyrrole†
Abstract
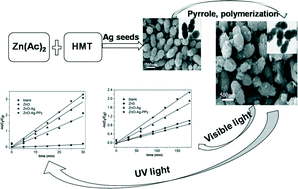
A fast reaction between zinc acetate and hexamethylenetetramine was carried out to produce peanut-shaped ZnO–Ag heterostructures in ethylene glycol/water mixed solvent using Ag nanoparticles as seeds and polyvinylpyrrolidone as surfactant. The Ag nanoparticles were well-dispersed on the ZnO particles, providing them with better photocatalytic performance in the decomposition of methylene blue than the pure ZnO. Furthermore, the introduction of a layer of polypyrrole coating on the ZnO–Ag surface enhanced the catalytic activity in two ways. On one hand, polypyrrole could protect the Ag from oxidation and being lost under both UV irradiation and visible light. On the other hand, polypyrrole could prominently increase the absorption of photons under the visible light.

 Please wait while we load your content...
Please wait while we load your content...