Morphology-mediated tailoring of the performance of porous nanostructured Mn2O3 as an anode material†
Abstract
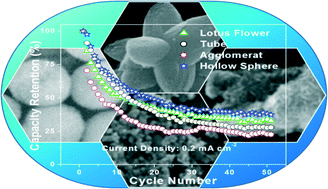
Tailoring of functional properties by varying the size and shape of porous nanostructured materials is an important frontier area of research. Herein, we report the successful synthesis of nanostructured Mn2O3 with desired 3D architectures such as porous hollow spheres, lotus shapes and tubular shapes, as well as aggregated nanoparticles, through the calcination of corresponding MnCO3 with the same architecture. Porous structures were formed upon evolution of CO2 during decomposition of the carbonate intermediate, and hollow structures were formed through a nonequilibrium interdiffusion process, i.e., the Kirkendall effect. The bare MnCO3 structures were synthesized using the chelating agents citric acid (CA), tartaric acid (TA), oxalic acid (OA), and ethylenediaminetetraacetic acid (EDTA), which mediated the growth of these MnCO3 structures by hydrothermal treatment of a precursor solution containing MnCl2, ammonium carbonate and chelating agent. A systematic evaluation of the effect of the morphology of the synthesized Mn2O3 on its performance as an anode material in Li-ion batteries reveals that the shape and the nature of pores of Mn2O3 strongly influence its Li-ion storage capacity. A superior specific capacity of 478 mAh g−1 is obtained for hollow spheres with 38% retention after 30 cycles compared to other shapes due its high accessible surface area and inner hollow architecture.

 Please wait while we load your content...
Please wait while we load your content...