Solvent-mediated phase transformation between two tegafur polymorphs in several solvents†
Abstract
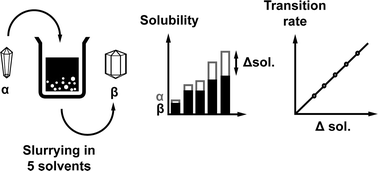
This paper describes a study of the solvent-mediated polymorphic transformation (SMPT) of the metastable α tegafur to the thermodynamically stable β tegafur in several solvents. Phase transformation in acetone, ethanol, i-propanol, toluene, and water at 22 °C was described using the solid-state kinetic model P2; the rate constants for this process were in the range from 0.028 min−1 to 0.0056 min−1. In all of the employed solvents, an induction time was observed. Kinetic, solubility and scanning electron microscopy data indicated that nucleation kinetics corresponded to a second-order power function and according to the kinetic model, the nuclei growth rate was constant in the examined SMPT. Surface nucleation was observed, and the possible nucleation mechanism was given. The phase transition rate depended linearly on the difference between the equilibrium solubilities of α and β tegafur in the respective solvent, i.e. supersaturation.

 Please wait while we load your content...
Please wait while we load your content...