Polymorphic and solvate structures of ethyl ester and carboxylic acid derivatives of WIN 61893 analogue and their stability in solution†
Abstract
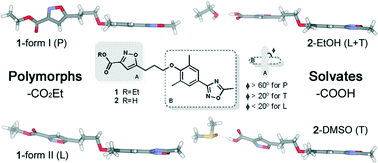
3-Ethyl ester- (1) and 3-carboxylic acid-isoxazole (2) derivatives of an antiviral drug analogue WIN 61893 were synthesized and characterized by X-ray crystallography and NMR spectroscopy. Crystallization experiments afforded two polymorphic structures for the ethyl ester derivative and two solvate structures for the carboxylic acid derivative based on their ability to form intermolecular hydrogen bonding interactions with the solvent molecules. The conformations of the derivatives depended greatly on the orientation of the planar isoxazole and phenyl-oxadiazole ring systems with respect to one another and were found to take up perpendicular, linear or tilted conformations. The carboxylic acid derivative was furthermore observed to undergo isoxazole ring cleavage by decarboxylation in DMSO solution and transforming into a β-keto nitrile ring-opening product over several days, whereas, the isoxazole ring of the ethyl ester derivative was not affected.

 Please wait while we load your content...
Please wait while we load your content...