Tuning of net architectures of Ni(ii) and Zn(ii) coordination polymers using isomeric organic linkers†
Abstract
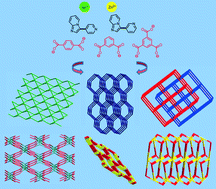
Solvothermal syntheses involving the hybrid ligands {3-pyridin-3-ylimidazo[1,2-a]pyridine (L1) and 3-pyridin-4-ylimidazo[1,2-a]pyridine (L2)}, aromatic polycarboxylates [1,4-benzenedicarboxylate (1,4-bdc), 1,3-benzenedicarboxylate (1,3-bdc), and 1,3,5-benzenetricarboxylate (1,3,5-btc)], and transition metal ions [Ni(II) and Zn(II) ions] afforded five new coordination polymers, namely, [Ni(1,4-bdc)(L1)(H2O)2]n (1), [Ni2(1,3-bdc)2(L1)2(H2O)]n (2), [Zn2(1,4-bdc)1.5(L1)(OH)]n (3), and [Zn2(1,3,5-btc)(L1)O0.5(H2O)0.5·0.5H2O]n (4), and [Zn2(1,3,5-btc)(L2)(OCOH)(H2O)]n (5). A diversity of net topologies was observed including a rare 2D 4-connected net with the point symbol of (44·63·8) in 1, a dia net in 2, a twofold interpenetrating pcu net in 3, a rare 3D (3,8)-connected binodal 3,8T35 net in 4, and sra and rtl nets in 5. Four different secondary building units were involved in the construction of the 3D nets. The three Zn(II) complexes exhibit photoluminescence.


 Please wait while we load your content...
Please wait while we load your content...