A new lead(ii) nanoporous three-dimensional coordination polymer: pore size effect on iodine adsorption affinity†
Abstract
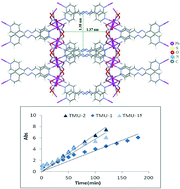
A three-dimensional (3D) nanoporous coordination polymer, [Pb(4-bpdb)(μ-NO3)(μ-SCN)]n·1.5CH3OH (TMU-15), was synthesized from the reaction of Pb(II) salt and 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene (4-bpdb) in a branched tube. Compound TMU-15 is stable for loading of guests. In this compound, we successfully loaded the porous crystals with I2 by suspending them in a solution of I2 in cyclohexane. The delivery of I2 from TMU-15 was performed in ethanol, a nonaromatic solvent, at room temperature and determined by UV/vis spectroscopy. The delivery and desorption rate of iodine from TMU-15 were also compared with those from analog compounds such as [Pb(4-bpdh)(NO3)2(H2O)]n (TMU-1) and [Pb(4-bpdh)(NO3)2]n (TMU-2).

 Please wait while we load your content...
Please wait while we load your content...