Crystal engineering with coordination compounds of NiII, CoII, and CrIII bearing dipicolinic acid driven by the nature of the noncovalent interactions†
Abstract
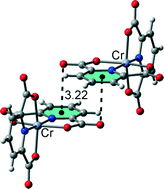
Five new proton transfer compounds, (Hapy)[Cr(pydc)2]·5H2O·[pydcH2] (1), (Hacr)[Cr(pydc)2]·2H2O (2), (H9a-acr)[Cr(pydc)2]·4.27H2O (3), [Co(pydc)(pydcH2)]·3H2O (4) and [Ni(pydcH)2]·H3O·2H2O (5) (where pydcH2 = pyridine-2,6-dicarboxylic acid; apy = 2-aminopyridine; acr = acridine and 9a-acr = 9-aminoacridine) have been synthesized and characterized by elemental analysis, IR spectroscopy and single crystal X-ray diffraction techniques. Crystallographic analysis revealed that compounds 1–5 have distorted octahedral geometries with pydc2− and pydcH− ions or pydcH2 coordinated as tridentate ligands to each metal ion through one oxygen atom of each carboxylate group and the nitrogen atom of the pyridine ring. Compounds 1–3 consist of cationic complexes where the dipicolinic acid is coordinated to the metal ion in the dianionic form, whereas 4 and 5 are neutral complexes. In 4, the acid is coordinated to the metal ion in the neutral form, which is quite rare. In the crystal structures of the different complexes, extensive O–H⋯O, N–H⋯O and C–H⋯O hydrogen bonds, as well as other noncovalent interactions involving aromatic rings (C–H⋯π, C–O⋯π and π⋯π stacking), were described and analysed by means of density functional theory (DFT) calculations since they play an important role in the construction of three-dimensional supramolecular frameworks.

 Please wait while we load your content...
Please wait while we load your content...