Spontaneous resolution of chiral bis-sulfoxides with asymmetric atropisomerism†
Abstract
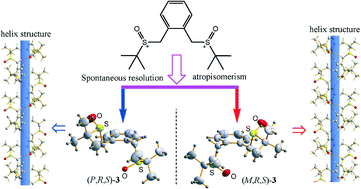
Restricted rotation of the ortho sulfinyl groups directs the trans ortho-substituted bis-sulfoxide (3) to adopt right-handed (P) or left-handed (M) conformation and thus produces symmetric atropisomeric (R,R)-3/(S,S)-3 and asymmetric atropisomeric enantiomers (R,S)-3, respectively. (R,S)-3 exhibits spontaneous resolution and crystallizes as a conglomerate (P,R,S)-3/(M,R,S)-3 with a homochiral supramolecular helix (P/M-helix) conformation.

 Please wait while we load your content...
Please wait while we load your content...