Efficient route to phase selective synthesis of type II silicon clathrates with low sodium occupancy†
Abstract
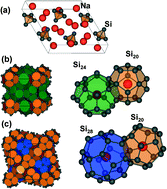
Phase selective synthesis of type II silicon clathrates from thermal decomposition of NaSi has previously been limited to small quantities due to the simultaneous formation of competing phases. In this work we show that the local sodium vapor pressure during the NaSi precursor decomposition is a critical parameter for controlling phase selection. We demonstrate synthesis techniques that allow us to tune the local Na vapor pressure, yielding type I or II clathrate products that are ≥90 wt.% phase pure. The “cold plate” reactor design discussed in this work maintains low Na vapor pressure during thermal decomposition of NaSi, thus yielding large scale, phase selective synthesis of type II silicon clathrates. The low Na vapor pressure maintained in this reactor is also shown to efficiently produce low Na (x ~ 1; NaxSi136) Si clathrate through Na sublimation. To further reduce sodium occupancy (x < 1), we demonstrate etching of NaxSi136 in HF/HNO3 solutions, which rapidly yields a clathrate product with reduced x. 29Si NMR and electron spin resonance (ESR) characterization validate the low Na occupancy of Si clathrate synthesized. The acid etch also selectively dissolves the type I silicon clathrate impurity phase, thereby enabling the synthesis of large quantities of phase pure type II silicon clathrate with low Na content.

 Please wait while we load your content...
Please wait while we load your content...