A facile synthesis of single crystal TiO2 nanorods with reactive {100} facets and their enhanced photocatalytic activity†
Abstract
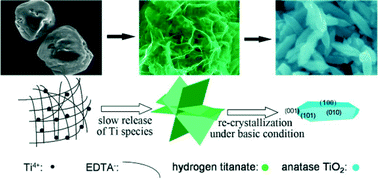
High-energy {100} faceted single crystal TiO2 nanorods were synthesized by a facile hydrothermal method. An interesting phase transition from the orthorhombic hydrogen titanate to anatase TiO2 was observed during the reaction process. A structural formation model of the TiO2 nanorods was proposed based on experimental evidence. The resultant {100} faceted TiO2 nanorods exhibited considerably enhanced photocatalytic activity towards degradation of organic pollutants and removal of heavy metal ions owing to the special one-dimensional structure with the reactive {100} facets, thus showing a great potential in the field of water treatment. At the same time, the synthetic route provided guidance for the synthesis of high-energy {100} facets using EDTA and urea as effective modifiers. This approach may be extended to synthesize other functional oxide crystals with well-defined morphologies and to increase the percentages of certain exposed facets.

 Please wait while we load your content...
Please wait while we load your content...