Constructing heterometallic frameworks with highly connected topology based on edge-to-edge hexanuclear lanthanide clusters†
Abstract
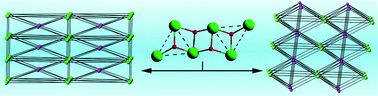
Hydrothermal reactions of Sm2O3 and CuI with 4-pyridin-4-ylbenzoic acid afford two heterometallic frameworks, namely, [Sm3Cu5I4L6(μ3-OH)2(OAc)(H2O)3]·ClO4·2H2O (1) (OAc = acetate) and [Sm6Cu14I12L14(μ3-OH)4(H2O)5]·2ClO4·8H2O (2). X-ray crystal structure analyses reveal that these two 3D pillared-layer heterometallic frameworks are both constructed from similar ribbon-like edge-to-edge hexanuclear Ln clusters. Interestingly, the {Sm6}1 cores in 1 and {Sm6}2 clusters in 2 are both connected by carboxyls of L ligands into 1D Ln-cluster organic chains along the a axis. In 1, such chains are linked by [CuL]− pillars and 1D stair-like [Cu4I4]n chains to give a pillared-layer 3D framework. Whereas in 2, these chains are extended by the [CuL]− pillars and [Cu12I12]n layers to form a 3D framework. Remarkably, to further stabilize the framework of 2, the [CuL]− pillars connect the {Sm6}2 clusters of neighbouring 1D Ln-cluster organic chains interpenetrating through the 11-ring windows of [Cu12I12]n layers. Topological analyses indicate that 1 and 2 possess a highly (6,14)-connected and a rare odd 11-connected topology, respectively. Furthermore, the IR, TGA, PXRD, UV-Vis spectra of 1 and 2 have also been studied.

 Please wait while we load your content...
Please wait while we load your content...