A compromise between competing forces dominating the diversity of aragonite structures
Abstract
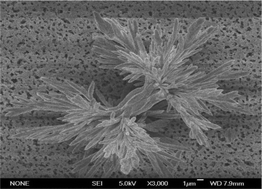
Material scientists have a strong interest in finding the reasons causing the diversity of material structures. Here, we show a different story in disclosing the mechanism dominating material structures. When we synthesize calcium carbonate particles at increased temperatures, we observe that the structures of the particles change with temperature. At a relatively low temperature (60 °C), dumbbell shaped particles are largely formed while straight particles are dominantly formed at a relatively high temperature (90 °C). By simulation, we find that the crystallite of aragonite is polarized after the adsorption of water molecules on its surface. The polarization is anisotropic, which makes the attachment of crystallites have different stabilities along different attaching directions. At an increased temperature, the attachment is easily destructed by Brownian motion which is dependent on temperature. Therefore, the adsorbing force provided by polarization competes with the destructive force induced by the Brownian motion. The compromise between these two competing forces dominates the stable structure of calcium carbonate particles. Hence, the compromise in competition between attractive forces and destructive forces leads to the diversity of aragonite structures, which indicates a new direction in discovering the diversity of material structures.

 Please wait while we load your content...
Please wait while we load your content...