Halogen bonding directed supramolecular assembly in bromo-substituted trezimides and tennimides†
Abstract
Reaction of isophthaloyl dichloride (I) with 2-amino-5-bromopyridine (BrO) and 2-amino-5-bromopyrimidine (26BrO) produces the trimeric (trezimide) and tetrameric (tennimide) imide-based macrocycles (BrIO)3 and (BrIO)4, and (26BrIO)3 and (26BrIO)4, respectively, in one-step and in modest yields. Macrocyclisation (forming 18-/24-membered rings) is facilitated by the imide hinge [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N(R)–C
C–N(R)–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O] (R = ortho-N-substituted-pyridine/pyrimidine), though competing reactions also lead to considerable oligomer/polymer formation. The macrocycles (having 3 or 4 Br atoms) lack classical strong hydrogen bonding donors (i.e. N–H, O–H), but contain numerous acceptors (i.e. Naromatic, C
O] (R = ortho-N-substituted-pyridine/pyrimidine), though competing reactions also lead to considerable oligomer/polymer formation. The macrocycles (having 3 or 4 Br atoms) lack classical strong hydrogen bonding donors (i.e. N–H, O–H), but contain numerous acceptors (i.e. Naromatic, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, arene) participating in halogen and weaker hydrogen bonding. Five crystal structures are analysed with the macrocycles typically aggregating through short C–Br⋯O
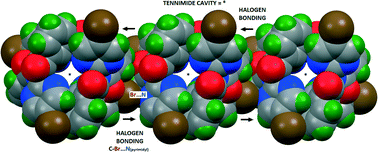
O, arene) participating in halogen and weaker hydrogen bonding. Five crystal structures are analysed with the macrocycles typically aggregating through short C–Br⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ccarbonyl and/or C–Br⋯Naromatic halogen bonds (Nc ≤ 0.90) and often associated with longer C–Br⋯H/π(arene) contacts. Of note, (BrIO)3 exhibits three types of Br⋯O
Ccarbonyl and/or C–Br⋯Naromatic halogen bonds (Nc ≤ 0.90) and often associated with longer C–Br⋯H/π(arene) contacts. Of note, (BrIO)3 exhibits three types of Br⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/π(arene) halogen bonds with Br⋯H and C–H⋯O contacts. The C–Br⋯N/O halogen bonds, in promoting overall aggregation, typically link macrocycles into 1-D halogen bonded chains.
C/π(arene) halogen bonds with Br⋯H and C–H⋯O contacts. The C–Br⋯N/O halogen bonds, in promoting overall aggregation, typically link macrocycles into 1-D halogen bonded chains.

 Please wait while we load your content...
Please wait while we load your content...