Cobalt-cluster-based coordination polymers with size-matching mixed ligands†
Abstract
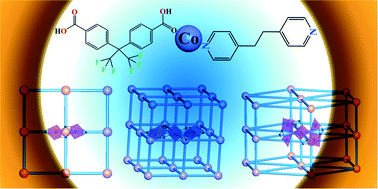
A series of mixed ligand cobalt coordination polymers built by V-shaped O-donor ligand and linear N-donor ligand, namely [Co(BPE)(L)] (1), [Co3(BPE)2(HL)2(L)2] (2), and [Co5(BPE)2(OH)2(L)4]·4DMA (3) (H2L = 4,4′-(hexafluoroisopropylidene)-bis(benzoic acid), BPE = 1,2-di(4-pyridyl)ethane, DMA = dimethylacetamide), was synthesized under hydrothermal/solvothermal conditions. Compound 1 displays a two-dimensional (2-D) double layer structure of {Co2} clusters bridged by BPE and L2− ligands. Compounds 2 and 3 exhibit three-dimensional (3-D) networks built by {Co3} (2) and {Co5} (3) clusters, respectively, interconnected by BPE and L2− ligands. Topological analysis indicates compound 2 has a doubly interpenetrated (412·63)-pcu (α-Po, 6-connected) network, whereas compound 3 possesses an 8-connected (36·418·53·6)-hex network. It has been found that the reaction media and reaction conditions play key roles for the formation of cobalt clusters in the self-assemblies of compounds 1–3.

 Please wait while we load your content...
Please wait while we load your content...