Two-step phase transformation of anatase to rutile in aqueous suspension†
Abstract
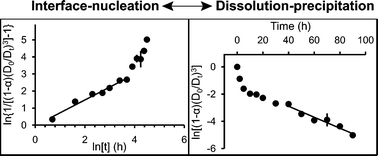
Kinetic data tracking the anatase to rutile phase transformation under acidic, hydrothermal conditions (200 °C, pH 1) are consistent with a two-step transformation mechanism. Fitting the experimental data using phase transformation models, interface–nucleation dominates the early stages of transformation and dissolution–precipitation dominates at later stages. During the first stage, the rate of transformation by interface–nucleation is consistent with a substantially higher number of nucleation sites, possibly generated via crystal growth by oriented aggregation. Characterization of the hydrothermally treated samples using HRTEM revealed that anatase crystal morphologies indicative of crystal growth by oriented aggregation are common. Furthermore, twinned rutile crystals, which are consistent with transformation of anatase twinned across the {112} to rutile twins by interface–nucleation, were also observed. At later stages, the kinetics of the anatase to rutile transformation are consistent with dissolution–precipitation, and the number concentration of rutile crystallites reaches a plateau. These results are consistent with a decrease in crystal growth by oriented aggregation as the anatase particle size increases and the dissolution of anatase and reprecipitation onto already existing rutile crystals due to the larger bulk energy and comparatively small size of anatase.
- This article is part of the themed collection: Nanocrystal growth via oriented attachment

 Please wait while we load your content...
Please wait while we load your content...