Low-temperature growth of spinel-type Li1+xMn2−xO4 crystals using a LiCl–KCl flux and their performance as a positive active material in lithium-ion rechargeable batteries†
Abstract
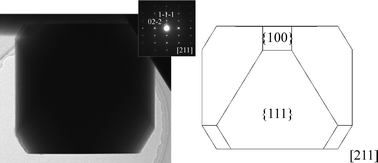
Low-temperature growth of idiomorphic spinel-type Li1+xMn2−xO4 (x = 0.09, 0.14) crystals was achieved by using a LiCl–KCl flux. The flux growth driven by rapid cooling resulted in truncated octahedral Li1+xMn2−xO4 crystals surrounded by both dominating {111} and minor {100} faces. The chemical compositions, sizes, and shapes of the Li1+xMn2−xO4 crystals could be tuned by simply changing the growth conditions. Among the various products, the crystals grown at a low temperature of 600 °C showed a small average size of 0.2 μm. The small Li1+xMn2−xO4 crystals grown at 600 °C showed better rate properties than the large crystals grown at 900 °C, when used as a positive active material in lithium-ion rechargeable batteries.

 Please wait while we load your content...
Please wait while we load your content...