Surface ligand mediated growth of CuPt nanorods†
Abstract
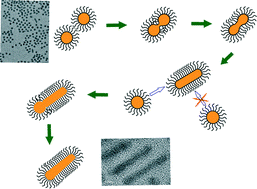
CuPt alloy nanorods have been synthesized via one dimensional assembly of randomly orientated nanocrystallites in the presence of hexadecanoic acid and hexadecylamine as surface ligands. When hexadecanoic acid was added into the synthetic system first followed by a second step of adding hexadecylamine, strands of ultrathin CuPt nanowires were produced. The roles of the amine and organic acid are discussed. A novel ligand mediated mechanism is proposed, in which the formation of a stable monolayer structure of the ligands is the driving force to guide the 1D growth of the alloy nanorods without the influence of the crystal orientation. Photocatalytic hydrogen production from water has been performed using CuPt nanorods as a cocatalyst, which has a higher production rate (234.08 μmol h−1 g−1) than that of Pt nanorods under the same conditions (~66.35 μmol h−1 g−1). Our results suggest that polycrystalline CuPt nanorods with a large amount of defects are probably promising cocatalyst for photocatalysis.

 Please wait while we load your content...
Please wait while we load your content...