Functionalized metal–organic framework MIL-101 for CO2 capture: multi-scale modeling from ab initio calculation and molecular simulation to breakthrough prediction†
Abstract
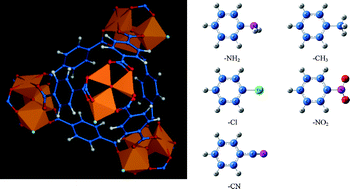
By synergizing ab initio calculation, molecular simulation and breakthrough prediction, we investigate CO2 capture in metal–organic framework MIL-101 functionalized by a series of groups (–NH2, –CH3, –Cl, –NO2 and –CN). CO2 uptake and isosteric heat in a low-pressure regime increase in the order of MIL-101 < MIL-101-CN < MIL-101-NO2 < MIL-101-Cl < MIL-101-CH3 < MIL-101-NH2. This order follows the strength of the binding energies between CO2 and the functional groups. However, the effect of the functional groups is marginal for N2 adsorption. In terms of the separation of a CO2/N2 mixture, CO2/N2 selectivity is enhanced by functionalization following the order of MIL-101 < MIL-101-CN < MIL-101-CH3 < MIL-101-NO2 < MIL-101-Cl < MIL-101-NH2. At an infinite dilution, the enhancement of CO2/N2 selectivity is 2.5 times. The predicted breakthrough time is extended by functionalization, and the longest breakthrough time in MIL-101-NH2 is 2 times that in MIL-101. Furthermore, the working capacity of CO2 increases by approximately 40%. This multi-scale modeling study suggests that CO2 capture in MIL-101 can be considerably improved by functionalization, in terms of CO2 capacity, CO2/N2 selectivity, breakthrough time and working capacity.

 Please wait while we load your content...
Please wait while we load your content...