Fast and mass synthesis of ZnS nanosheets via an ultra-strong surface interaction
Abstract
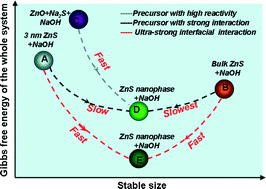
In this report, we develop a strategy for the fast and mass synthesis of ZnS nanosheets based on the theory of thermodynamically stable nanophase. In an NaOH–NaI eutectic environment where an ultra-strong surface interaction exists, both bulk and 3 nm ZnS can be quickly converted to nanosheets with the same thickness. We use X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to testify that the nanosheet products from either the bulk or 3 nm ZnS are identical structurally and morphologically. The structure of the nanosheet is studied thoroughly and the function of the eutectic system towards the formation of an intense surface interaction as well as the reduction of the conversion time is theoretically analyzed. We further demonstrate that the system of thermodynamic control is insusceptible to the small changes in the starting chemical components and the thermodynamic conversion approach can be extended to doping other metal ions in the ZnS nanosheet. In addition, one significant merit for the thermodynamically stable system is that the product can be mass produced and the time required can be greatly reduced. We proposed that the direct conversion to the thermodynamically stable nanophase material has a potential advantage in industrial mass and fast production.

 Please wait while we load your content...
Please wait while we load your content...