The influence of strong and weak hydrogen bonds on the solid state arrangement of hydroxy-containing boron subphthalocyanines†
Abstract
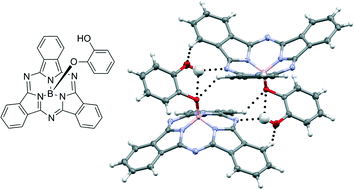
We have investigated the occurrence of hydrogen bonding in boron subphthalocyanine (BsubPc) derivatives by synthesizing a series of four hydroxy-containing BsubPcs and studying their solid state arrangements using X-ray diffraction. The series of derivatives comprises hydroxy-BsubPc (HO-BsubPc) as well as para-, meta-, and ortho-hydroxy-phenoxy-BsubPc (p-, m- and o-HOPhO-BsubPc). Each solid state arrangement demonstrated both strong (O–H⋯N, O–H⋯O) and weak (C–H⋯O, C–H⋯N) hydrogen bonds, with the structure for o-HOPhO-BsubPc including examples of bifurcated hydrogen bonds. Many of the C–H⋯O interactions were found to occur alongside notable perturbations to the expected intra- and intermolecular geometry of BsubPcs, providing structural evidence for the significance of weak C–H⋯O bonds. We observed that the imine nitrogen atom of the BsubPc moiety is a reliable hydrogen bond acceptor, and in most arrangements, the hydroxy moiety participated in hydrogen bonds both as a donor and an acceptor. The presence of hydrogen bonds in addition to other intermolecular interactions (π–π and CH–π, which are common in BsubPc solid state arrangements) facilitates the arrangement of BsubPc into two- and three-dimensional networks of closely associated molecules.

 Please wait while we load your content...
Please wait while we load your content...