Transition metal-directed assembly of diverse coordination polymers based on multifunctional ligand 2,4-dichloro-5-sulfamoylbenzoic acid†
Abstract
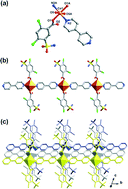
Four new coordination polymers based on Co2+/Cd2+/Zn2+/Cu2+, Co(bpy)(H2sba)2(H2O)2 (1), Cd(bpy)(Hsba)H2O (2), Zn(bpy)(Hsba) (3), [Cu6(bpy)5(H2ssa)6(H2O)2]·5H2O (4), (bpy = 4,4′-bipyridine, H3sba = 2,4-dichloro-5-sulfamoylbenzoic acid, H4ssa = 4-chloro-5-sulphamoylsalicylic acid) have been successfully synthesized using the mild hydrothermal method with similar reaction conditions. X-ray diffraction analyses reveal that compound 1 exhibits a 1D linear chain formed by the linkage of Co centres and bpy ligands. Compound 2 shows a 2D window-shaped layer with 44·62sql topology. Compound 3 is a 3D framework containing a new topological prototype with a symbol of 65·8. Compound 4 is characteristic of a 3D honeycomb lattice of hexagonal channels which are occupied by H2ssa2− ligands. Left- and right-handed helical chains with equal numbers in the framework are observed, which are constructed by repeating neutral trinuclear units of [Cu3(H2ssa)3]. Worthy to mention here, when Cu2+ ions were introduced into the present reaction system, a new ligand H2ssa2− was obtained in situ by the hydrolysis of chlorine in H3sba, which represents the first example of hydrolysis of chlorine either in coordination polymers and/or in hydrothermal synthesis. Moreover, the luminescent properties for compounds 2 and 3 as well as the H3sba ligand, and the magnetic properties of compound 4 have been investigated in the solid state.

 Please wait while we load your content...
Please wait while we load your content...