Whewellite, CaC2O4⋅H2O: structural study by a combined NMR, crystallography and modelling approach†
Abstract
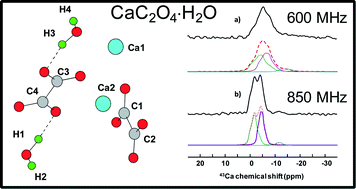
Multinuclear solid state NMR (including 43Ca NMR), in combination with DFT calculations, is applied to the study of the crystal structure of whewellite, CaC2O4⋅H2O. This particular hydrated calcium oxalate is of paramount importance as it corresponds to a major phase present in urinary stones. 43Ca MAS NMR experiments and GIPAW calculations were performed in order to further refine neutron diffraction data. The sensitivity of 43Ca NMR as a structural probe is demonstrated. This is the first step for the full description of calcium oxalates at the DFT level and the characterization of interfaces between these biomineral phases and organic phases.
- This article is part of the themed collection: NMR crystallography

 Please wait while we load your content...
Please wait while we load your content...