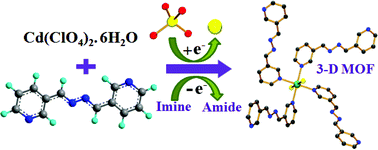
Three new metal–organic hybrids have been synthesized in a reaction of Cd(II) with 1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene (3-bpdb) and disodium succinate (Na2suc). All three compounds, i.e., {[Cd(3-bpdb)(Cl)]·ClO4}n (1), {[Cd(3-bpdb)3(H2O)2]·(3-bpdb)(ClO4)2}n (2) and {[Cd(3-bpdb)(suc)(H2O)2]·(H2O)2}n (3) are characterized by single-crystal X-ray diffraction and other physicochemical methods. Structure determination reveals that 1 shows an α-polonium type 3D coordination network created by an exactly perpendicular Cl–Cd–Cl linkage. The framework of 1 contains 1D channels, which are filled with ClO4−. Compound 2 shows a 1D coordination structure with two bridging and two pendent 3-bpdb ligands. These pendent ligands are involved in H-bonding, π–π and C–H⋯π interactions with its coordinated water molecules and lattice 3-bpdb ligands, to form the 3D supramolecular structure. Compound 3 is a 2D 4-connected net with succinate and the 3-bpdb ligand and extended to 3D supramolecular architecture by H-bonding and π–π interactions. During the syntheses, an in situ chemical transformation of perchlorate to chloride has occurred along with the oxidation of imine to amide and the chlorides so produced are found integrated in compound 1, which facilitates the oxidation of imine in a very unprecedented way.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...