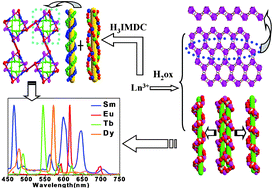
Three series of two-dimensional and three-dimensional lanthanide coordination polymers {[Ln(ox)1.5(H2O)3]·mH2O}n [Ln = Ce (1), m = 2 and Pr (2), m = 3, H2ox = oxalic acid], {[Ln(ox)2(H3O)]·EtOH·3H2O}n [Ln = Nd (3), Sm (4), Eu (5), Gd (6), Tb (7), Dy (8), Ho (9), Er (10) and Yb (11), EtOH = ethanol] and {[Ln2(IMDC)2(H2O)3]·mH2O}n [Ln = Sm (12), Eu (13), Gd (14), Tb (15) and Dy (16), where m = 2.25 except for 15 where m = 1.75, H3IMDC = imidazole-4,5-dicarboxylic acid] have been synthesized under hydrothermal conditions. All the coordination polymers have been characterized by elemental analysis, IR spectroscopy and X-ray single-crystal diffraction. Coordination polymers 1–11 exhibit two different structural types: the 2-D structure of coordination polymers 1–2 are a (6,3)-connected hcb network, coordination polymers 3–11 appear as a 3-D diamond structure with 66-network topology. Coordination polymers 12–16 are 3-D structures constructed from the bridged carboxyl group of IMDC3−, in which IMDC3− bridged the DNA-like double-helix line and the triple-helix line, intertwined and connected into a 1-D chain and then conformed into a 3-D structure along the ab plane. The different structures of the two dicarboxylate ligands (oxalic acid as a flexible ligand and imidazole-4,5-dicarboxylic acid as a rigid planar ligand) were described and discussed. Furthermore, the luminescent properties in the visible region and the fluorescence lifetimes of the coordination polymers exhibited the characteristic transitions of the corresponding lanthanide ions. It was noted that coordination polymers 5 and 7 have long solid-state fluorescence lifetimes of 824.84 and 733.87 μs, respectively. The NIR emission spectra of the Nd (3), Sm (4 and 12), Dy (8 and 16) and Yb (3) coordination polymers in the solid-state were measured. The singlet excited state (30 842 cm−1 for oxalic acid, 32 258 cm−1 for H3IMDC) and the lowest triplet state energy level (23 753 cm−1 for oxalic acid and 22 371 cm−1 for H3IMDC) of the H2ox and H3IMDC ligand were calculated on the basis of the UV-Vis absorbance edges of the ligand and the phosphorescence spectrum of the Gd(III) coordination polymers 6 and 14 at 77 K, respectively. The relationship between the lowest triplet state energy level of the two dicarboxylate ligands and lowest resonance energy levels of the Sm(III), Eu(III), Tb(III) and Dy(III) ions were described and discussed. The results showed that the energy transfer from the oxalic acid ligands is much more effective than H3IMDC.

 Please wait while we load your content...
Please wait while we load your content...