The solvates and salt of antibiotic agent, nitrofurantoin: structural, thermochemical and desolvation studies†
Abstract
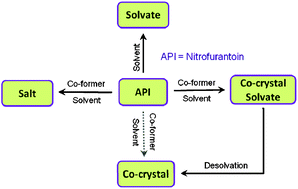
The ability of an antibacterial agent, nitrofurantoin (NF), to form molecular complexes with various pyridyl bases and 4-aminobenzamide are investigated. Five solvates with the solvents pyridine (PYR, 1 : 1), 2/3/4-picolines (2/3/4PIC, 1 : 1), 3-picoline + water (3PIC, H2O, 1 : 1 : 1), two co-crystal solvates involving 2-pyridone (2PYR) or 4-aminobenzamide (4ABM) with a solvent acetonitrile (ACN) and a salt with 4-dimethylaminopyridine (DMAP) were identified and characterized. Crystal structure analysis revealed that the N–H⋯N heterosynthon between imide N–H and pyridyl–N are predominantly observed in the solvates involving NF, whereas in a salt (NF-4DMAP), the +N–H⋯N− heterosynthon is noted. Thermal analysis established the stability and confirmed molar ratios of the reported solvates. Desolvation of NF solvates, NF-PYR, NF-2PIC, NF-3PIC-H2O, NF-3PIC, NF-4PIC, yielded the anhydrous NF (β-form). Interestingly, co-crystal solvates (NF-2PYR-ACN and NF-4ABM-ACN) upon desolvation produced novel anhydrous co-crystals. The results suggest that co-crystal solvates could be an alternative route to prepare anhydrous co-crystals that offer further avenues for expanding the new solid forms involving APIs.

 Please wait while we load your content...
Please wait while we load your content...