Polyhedral Cu2O particles: shape evolution and catalytic activity on cross-coupling reaction of iodobenzene and phenol†
Abstract
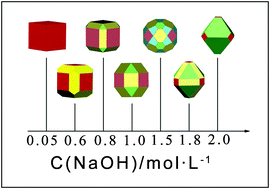
In this study, we have successfully achieved a systematic and precise shape control of Cu2O nano- and microcrystals in alkaline aqueous solution using

 Please wait while we load your content...
Please wait while we load your content...