pH-assisted crystallization of Cu2O: chemical reactions control the evolution from nanowires to polyhedra†
Abstract
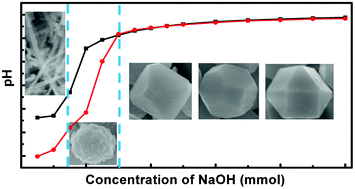
This work reports the chemical reaction-controlled crystallization of Cu2O with systematic shape evolution from nanowires, through nanoparticle-aggregated spheres and octahedra to truncated octahedra and cuboctahedra by a two-step “precursor formation–crystallization” process. The results reveal that the shape, size, composition variation and overall reaction mechanism were mostly determined by the pH-dependent precursor species Cu2(OH)3NO3, Cu(OH)2 and Cu(OH)42−. The addition of specific amounts of OH− to the solution is the key to adjusting the reduction and complexation reactions that control the crystallization of Cu2O; high pH enhanced the reducing power of starch and the complexation ability of OH−. Cu2O or Cu can be selectively crystallized under alkaline or acidic conditions due to the pH-dependence of the reduction reaction. The intriguing results show that the size of truncated octahedra first increases, then decreases with increasing pH owing to the competition between the complexation and redox reactions. The results demonstrate that control of pH-dependent chemical reactions is an effective way to adjust the crystallization of oxides and their electrochemical activity.

 Please wait while we load your content...
Please wait while we load your content...