One-pot preparation and enhanced photocatalytic and electrocatalytic activities of ultralarge Ag/ZnO hollow coupled structures†
Abstract
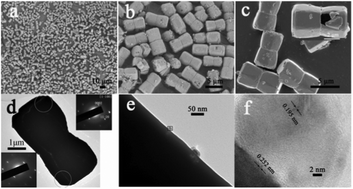
Inorganic nanostructures with hollow interior space have aroused great research interests in recent years. However, most reported works so far possess relatively small particle sizes since the hollow materials with large interior cavities and thin walls are low in mechanical strength and prone to collapse. In this work, we report an ultralarge-sized Ag/ZnO hollow coupled structure, which has a diameter as large as 3–4 μm and length reaching 6–8 μm. In particular, the coupled structure is built from two identical single-crystal truncated pyramids which show highly uniform morphologies with good and complete shells, indicating strong enough structures and well preserved hollow interior spaces. On the basis of our investigations, the time-dependent hollowing can be well attributed to the Ostwald ripening process. The photocatalytic degradation of Rhodamine B shows that the obtained Ag/ZnO hollow-structured composite exhibits higher photodegradation efficiency than that of the ZnO product. The Ag/ZnO hollow nanostructures modified electrode also displays excellent electrocatalytic response for the detection of hydrogen peroxide (H2O2), exhibiting about 18 times higher sensitivity than the bare electrode. The approach of one-pot preparation provides a potential method to fabricate other oxide or metal/oxide hollow structures.

 Please wait while we load your content...
Please wait while we load your content...