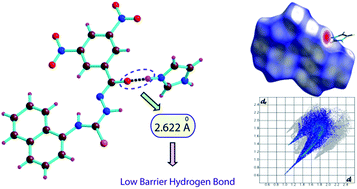
The complexes (1–3) of an amidothiourea receptor L with three different types of organic bases have been synthesized, and their crystal structures were solved using single crystal X-ray diffraction data. A detailed structural analysis of the complexes reveals that the complexes are primarily stabilized by a strong hydrogen bonding interaction between the amide oxygen of the deprotonated receptor and N–H proton of the protonated bases. Close inspection of the hydrogen bond parameters of the complexes shows that the receptor significantly forms a low barrier hydrogen bond with imidazole and hexamine, whereas no obvious low barrier hydrogen bond has been found in case of triethylamine. The Hirshfeld surface and fingerprint plot analysis of the complexes have been carried out, and reveal that the complexes are stabilized O–H⋯O, C–H⋯O, N–H⋯S and N–H⋯O, hydrogen bonds. Additionally, the nitro groups of the receptor are involved in different types of charge transfer or electron donor acceptor interactions, which also have a prominent effect in the solid state stabilization of the complexes (1–3).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...