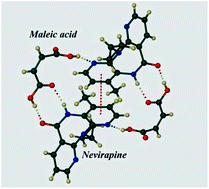
Synthesis and physicochemical characterization of co-crystals of the antiretroviral drug nevirapine (NV) with the pharmaceutically acceptable co-formers saccharin, rac-tartaric acid, maleic acid, glutaric acid and salicylic acid are reported for the first time. The respective stoichiometric NV : co-former ratios are 2 : 1, 1 : 1, 1 : 1, 1 : 1 and 2 : 1. In the 1 : 1 co-crystals, the predicted R22(8) NV(amide)–carboxylic acid supramolecular synthon occurs, whereas in the 2 : 1 species, the co-former is H-bonded to a centrosymmetric NV dimer formed via the R22(8) amide–amide synthon. Thermal analysis of three co-crystals revealed the unusual phenomenon of precipitation of NV from the melt. Co-crystallization of NV with maleic acid led to the highest (∼fivefold) increase in the aqueous solubility of the drug.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...