Structural systematics and conformational analyses of a 3 × 3 isomer grid of nine N-(tolyl)pyridinecarboxamides and three chlorinated relatives†
Abstract
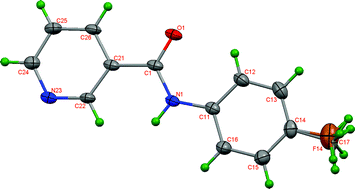
A 3 × 3 isomer grid of nine N-(tolyl)pyridinecarboxamides (NxxM, x = para-/meta-/ortho-) integrating crystal structure analyses, ab initio calculations (gas phase and PCM-SMD solvated in CH2Cl2, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds, whereas the six NoxM/5-Cl-NoxM structures exhibit intramolecular N–H⋯N interactions influencing co-planarity. The NmmM isomer is isomorphous and isostructural with its bridge-flippedMmm isomer providing a rare case of isostructuralism between such isomers. Our objectives are to (a) understand correlations between the p-/m-/o-N/CH3 substituent permutations with solid state molecular conformations and supramolecular aggregation, (b) explain the influence of molecular conformation on inter/intramolecular interactions, (c) correlate physico-chemical properties and (d) compare and rationalise differences between crystal and calculated structures.
C hydrogen bonds, whereas the six NoxM/5-Cl-NoxM structures exhibit intramolecular N–H⋯N interactions influencing co-planarity. The NmmM isomer is isomorphous and isostructural with its bridge-flippedMmm isomer providing a rare case of isostructuralism between such isomers. Our objectives are to (a) understand correlations between the p-/m-/o-N/CH3 substituent permutations with solid state molecular conformations and supramolecular aggregation, (b) explain the influence of molecular conformation on inter/intramolecular interactions, (c) correlate physico-chemical properties and (d) compare and rationalise differences between crystal and calculated structures.

 Please wait while we load your content...
Please wait while we load your content...