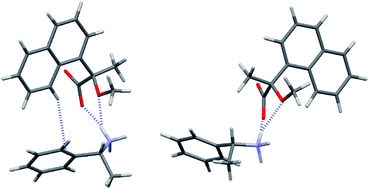
This paper is the first to report the structures of crystalline diastereomeric salts 8 and 9 prepared from (R)-2-methoxy-2-(1-naphthyl)propanoic acid [(R)-MαNP acid, (R)-1] and (S)-1 with (R)-1-phenylethylamine [(R)-PEA, (R)-7], respectively. These crystal structures helped elucidate a novel chiral recognition mechanism characteristic of MαNP salts. The less-soluble diastereomeric salt 8 prepared from (R)-1 and (R)-7 formed an ammonium–carboxylate ion pair by means of a methoxy-assisted salt bridge and an aromatic CH⋯π interaction. The more-soluble diastereomeric salt 9 prepared from (S)-1 and (R)-7 formed an ion pair by a methoxy-assisted salt bridge in which the 1-naphthyl and phenyl groups did not overlap. Instead, salt 9 formed a close ion pair by means of a salt bridge, a CH⋯O hydrogen bond, and a π⋯π interaction. These crystal structures suggest that the molecular length from the MαNP plane containing the carboxy and methoxy groups is critical to the crystallisation of diastereomeric salts. The crystal packing in both salts was investigated with regard to the weak interactions (i.e., salt bridges, NH⋯O and CH⋯O hydrogen bonds, and aromatic CH⋯π, CH⋯π, and π⋯π interactions). Finally, diastereomeric amides 11 and 12 were prepared from (S)-2-methoxy-2-(2-naphthyl)propanoic acid [(S)-MβNP acid, (S)-2] and (R)-2 with (S)-1-(1-naphthyl)ethylamine [(S)-10]. The solution-phase structures of the MβNP amides, and their separation, was investigated by NMR spectroscopy and high-performance liquid chromatography (HPLC). The less-stereochemically demanding and longer 2-naphthyl group made the MβNP amide more flexible and less polar than the MαNP amide. Acid 2 was more efficient than acid 1 in separating amides 11 and 12.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...